Alkanes are a form of hydrocarbons with a general formula CnH2n+2
Alkanes are made up of carbon chains with only single covalent bonds. (C-C)
As they have only C-C covalent bonds in the carbon chain they are called saturated.
Saturated hydrocarbons have no ‘spare bonds’ (no C=C or CΞC type bonding)
Bromine Water doesn’t decolourise with alkanes as it has no spare bonds.
Alkanes don’t form polymers again as it has no spare bonds
They burn cleanly in air producing carbon dioxide and water.
You need to know the names of the first four Alkanes these are: Methane, ethane, propane and butane.
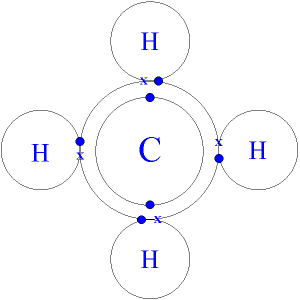
Methane CH4 (Dot-and-cross diagram)
Ethane C2H6
Propane C3H8
Butane C4H10



No comments:
Post a Comment